Answer:
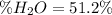
Step-by-step explanation:
Hello!
In this case, since the percent water is computed by dividing the amount of water by the total mass of the hydrate; we infer we first need the molar mass of water and that of the hydrate as shown below:
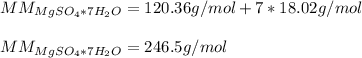
Thus, the percent water is:
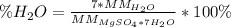
So we plug in to obtain:
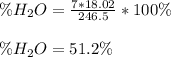
Best regards!