Answer:
0.67atm is the new pressure of the gas
Step-by-step explanation:
To find one condition of a gas when pressure, volume and absolute temperature change we must use combined gas law:
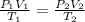
Where P is pressure, V is volume and T is absolute temperature of 1, initial state and 2, final state of the gas.
Replacing:
P₁ = 1.12atm
V₁ = 156mL
T₁ = 28.1°C + 273.15K = 301.25K
P₂ = ?
V₂ = 312mL
T₂ = 87.2°C + 273.15 = 360.35K
1.12atm*156mL / 301.25K = P₂*312mL / 360.35K
P₂ = 0.67atm is the new pressure of the gas