Answer:
CeO₂
Step-by-step explanation:
Hello!
In this case, since we are given the mass of both cerium and the cerium oxide, we can first compute the moles of cerium and the moles of oxygen as shown below:
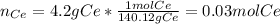
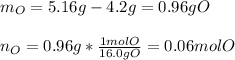
Now, we simply divide each moles by 0.03 as the fewest moles in the formula to obtain the simplest formula (empirical formula) of this oxide:
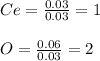
Thus, the formula turns out:
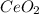
Regards!