Final answer:
After 24.0 hours, which equals four half-lives of the radioisotope technetium-99 with a half-life of 6.00 hours, only 1.25 mg of the original 20.0 mg dose would remain.
Step-by-step explanation:
The question is related to the decay of the radioisotope technetium-99 used as a radiotracer. Given that technetium-99 has a half-life of 6.00 hours, we can calculate the remaining amount of a 20.0 mg dose after 24.0 hours (which equals 4 half-lives) using the formula for exponential decay.
To find out how much of the radioisotope remains after a certain number of half-lives, we use the formula:
Remaining amount = Initial amount ×
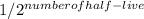
For technetium-99 after 24 hours:
- Initial amount = 20.0 mg
- Number of half-lives = 24 hours / 6 hours per half-life = 4
- Remaining amount = 20.0 mg × (1/2)4 = 20.0 mg × 1/16
- Remaining amount = 1.25 mg
Therefore, after 24.0 hours, 1.25 mg of the original 20.0 mg dose of technetium-99 would remain in the patient's body.