Answer: Because Kinetic energy depends on temperature and not mass
Step-by-step explanation:
Average kinetic energy is defined as the average of the kinetic energies of all the particles present in a system. It is determined by the equation:
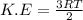
where K.E = Kinetic energy
R= gas constant
T= temperature in kelvin
It is visible that kinetic energy is dependent on the temperature of the system and not on the mass of the system. Thus particles with different masses have the same kinetic energy when at the same temperature