Answer:organic compound is : C₄H₈
Explanation: The equation of the reaction of the organic compound burning with excess oxygen gives us the general equation as
C xHy + 1/2(x+ y/2 )O₂ → xCO₂ + y/2H₂O
We first find the number of moles of CO₂ and H₂O
Number of moles of CO₂= Mass/ molar mass
= 17.6g/ 44g/mol ( 12+ 16 x2)
=0.4 moles
Number of moles of H₂O= Mass/ molar mass
= 7.2g/ 18g/mol ( 1 X 2+ 16)
=0.4 moles
From the reaction , we can see that
x = 0.4
and
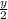 = 0.4 such that y= 0.4 x 2= 0.8
= 0.4 such that y= 0.4 x 2= 0.8
Their ratios become
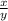 =
=
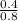 =
=
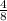
Therefor the organic compound CxHy = C₄H₈