Final answer:
To assign oxidation numbers to each specified element in the various compounds, we apply oxidation rules considering the states of common elements such as hydrogen and oxygen and balance them so that the sum equals the net charge of the molecule or ion.
Step-by-step explanation:
To assign oxidation numbers to the specified element in each of the following compounds, we use a set of rules including the oxidation state of hydrogen (+1), oxygen (-2), and the fact that the sum of oxidation numbers must equal the charge of the molecule or ion.
a) S in H2SO3: Sulfur (S) has an oxidation number of +4, since each hydrogen is +1 (total of +2) and each oxygen is -2 (total of -6) and the sum has to be zero for a neutral compound.
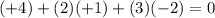 .
.
b) S in MgSO4: Sulfur (S) has an oxidation number of +6, with Mg being +2 and each oxygen -2. The sulfate ion (SO42-) has to equal -2 overall, so +6 for sulfur balances with -8 from oxygen.
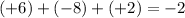 (charge of ion).
(charge of ion).
c) S in K2S: Sulfur has an oxidation number of -2, since potassium (K) is +1 and there are two K atoms for a total charge of
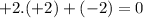 .
.
d) Cu in Cu2S: Copper (Cu) has an oxidation number of +1, as there are two copper atoms and one sulfur with
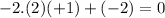 .
.
e) Cr in Na2CrO4: Chromium (Cr) has an oxidation number of +6. Sodium (Na) is +1 and oxygen is -2. The sum must equal -2 because of the two sodium ions:
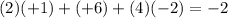 .
.
f) N in HNO3: Nitrogen (N) has an oxidation number of +5. Hydrogen is +1 and oxygen -2, and the sum must be zero.
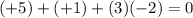 .
.
g) C in (HCO3)-: Carbon (C) has an oxidation number of +4. The bicarbonate ion has a -1 charge,
hydrogen is +1 and oxygen is
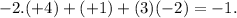
h) N in (NH4)+: Nitrogen (N) has an oxidation number of -3. Each hydrogen is +1 (total of +4) and the sum must equal +1 for
this ion.
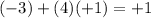 .
.