Answer:
Ration of internal energy of hydrogen to the internal energy of helium is equal to

Step-by-step explanation:
As we know
degree of freedom of hydrogen is 5
Degree of freedom of helium is 3
Internal energy of hydrogen
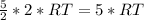
Internal energy of helium
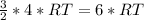
Ration of internal energy of hydrogen to the internal energy of helium is equal to
