Answer:
1/52
Explanation:
We are given that
Total number of cards=52
Number of cards of 4 of spade=1
We have to find the probability of choosing the "4 of spades".
We know that
Probability, P(E)=
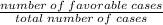
Using the formula
The probability of getting 4 of spade=
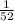
Hence, the probability of choosing the "4 of spades"=1/52