Final Answer:
The equilibrium constant (Kc) for the reaction is 0.01202.
Step-by-step explanation:
The reaction is:
2HI (g) <=> H
 (g) + I
(g) + I
 (g)
(g)
The equilibrium constant expression for this reaction is:
Kc = [H
 ][I
][I
 ] / [HI
] / [HI
![]^2](https://img.qammunity.org/2024/formulas/chemistry/high-school/u5mmeltfhr8ch9frxnzsa3x8b5jlss45wc.png)
where:
[H
 ] is the concentration of H
] is the concentration of H
 (g) at equilibrium
(g) at equilibrium
[I
 ] is the concentration of I
] is the concentration of I
 (g) at equilibrium
(g) at equilibrium
[HI] is the concentration of HI (g) at equilibrium
We can calculate the equilibrium concentrations of HI, H2, and I2 using the following ICE table:
HI H
 I
I

Initial 0.400 M 0.00 M 0.00 M
Change -0.072 M +0.036 M +0.036 M
Equilibrium 0.328 M 0.036 M 0.036 M
Now we can plug the equilibrium concentrations into the equilibrium constant expression to solve for Kc:
Kc = ([0.036
![]^2](https://img.qammunity.org/2024/formulas/chemistry/high-school/u5mmeltfhr8ch9frxnzsa3x8b5jlss45wc.png) ) / (0.328
) / (0.328
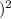
Kc = 0.01202.
Therefore, the equilibrium constant (Kc) for the reaction is 0.01202.