Answer: The average atomic mass of naturally occurring silicon is 28.08
Step-by-step explanation:
Mass of isotope Si-28 = 27.9769265327
% abundance of isotope Si-28 = 92.2297% =
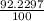
Mass of isotope Si-29 = 28.97649472
% abundance of isotope Si-29 = (4.6932)% =
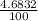
Mass of isotope Si-30 = 29.97377022
% abundance of isotope Si-30 = (3.0872)% =
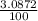
Formula used for average atomic mass of an element :
![A=\sum[(27.9769265327* (92.2297)/(100))+(28.97649472* (4.6832)/(100))+(29.97377022* (3.0872)/(100))]](https://img.qammunity.org/2022/formulas/chemistry/college/f0hnyklhodssji62cpcag750kxl2nuu2zk.png)
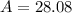
Therefore, the average atomic mass of naturally occurring silicon is 28.08