Answer:
1200 Sm^2mol^-1
Step-by-step explanation:
Given data :
conductivity of water ( kwater ) = 76 mS m^-1 = 0.076 Sm^-1
conductivity of kcl (aq)( Kkcl ) = 1.1639 Sm^-1
Kkcl = 1.1639 - 0.076 = 1.0879 Sm^-1
Resistance = 33.21 Ω
where conductivity can be expressed as =
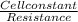
hence cell constant = conductivity * Resistance
= 1.0879 * 33.21 = 36.13m^-1
conductivity of CH3COOH ( kCH3COOH ) = 36.13 / 300
= 0.120 Sm^-1
Determine the molar conductivity of acetic acid
= ( kCH3COOH * 1000 ) / C
C = 0.1 mol dm
= (0.120 * 1000) / 0.1 = 1200 Sm^2mol^-1