Answer:
The height of her friend is 4.826 feet.
Explanation:
The length of the shadow (
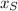 ), measured in feet, is directly proportional to the height of the person (
), measured in feet, is directly proportional to the height of the person (
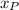 ), measured in feet. That is:
), measured in feet. That is:
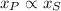
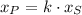 (1)
(1)
Where
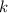 is the proportionality constant, no unit.
is the proportionality constant, no unit.
We can eliminate this constant by constructing this relationship:
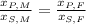 (2)
(2)
Where M and F represents Melody and Melody's friend. If we know that
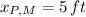 ,
,
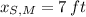 and
and
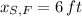 , then the height of his friend is:
, then the height of his friend is:
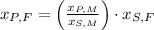
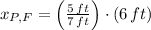
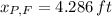
The height of her friend is 4.826 feet.