Answer: Molecular mass of
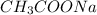 is 82 g
is 82 g
Step-by-step explanation:
Molecular mass (M) is defined as the mass in grams of 1 mole of a substance.
S.I Unit of Molar mass is gram per mole and it is represented as g/mol.
Atomic Mass of Carbon (C) = 12 g
Atomic Mass of hydrogen (H) = 1 g
Atomic Mass of oxygen (O) = 16 g
Atomic Mass of sodium (Na) = 23 g
Molecular mass of
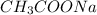 = 12(1)+1(3)+12(1)+16(2)+23(1) = 82 g
= 12(1)+1(3)+12(1)+16(2)+23(1) = 82 g