The new pressure : P₂ = 1038.39 mmHg
Further explanation
Given
1.5 L container at STP
Heated to 100 °C
Required
The new pressure
Solution
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure).
So P₁ = 1 atm = 760 mmHg
T₁ = 273 K
T₂ = 100 °C+273 = 373 K
Gay Lussac's Law
When the volume is not changed, the gas pressure is proportional to its absolute temperature
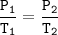
Input the value :
P₂=(P₁.T₂)/T₁
P₂=(760 x 373)/273
P₂ = 1038.39 mmHg