Final answer:
The half-life of the reaction is 4.3 x
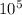 seconds and the concentration of ethyl chloride after 4 half-lives is 0.0048 M.
seconds and the concentration of ethyl chloride after 4 half-lives is 0.0048 M.
Step-by-step explanation:
The decomposition of ethyl chloride into ethylene and HCl is a first-order reaction with a rate constant of 1.6 × 10-6
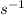 . To find the half-life of the reaction, we can use the formula:
. To find the half-life of the reaction, we can use the formula:
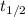 = ln(2) / k
= ln(2) / k
Substituting the given value of k, we get:
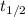 = ln(2) / 1.6 × 10-6 s-1 = 4.3 x 105 seconds.
= ln(2) / 1.6 × 10-6 s-1 = 4.3 x 105 seconds.
To find the concentration of ethyl chloride after 4 half-lives, we can use the formula:
final concentration = initial concentration ×
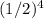
Substituting the given initial concentration, we get:
final concentration = 0.077 M ×
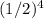 = 0.077 M × 0.0625 = 0.0048 M.
= 0.077 M × 0.0625 = 0.0048 M.