Step-by-step explanation:
Remember Newton's Second Law.
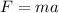
If the force acting on both bikers is the same, we can look at the relationship between acceleration and mass.
If Biker 1 has a mass of 10kg and Biker 2 has a mass of 20kg, and both are being acted upon by a force of 100 N, let's see what that looks like.
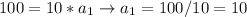
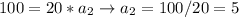
So, given the same force, an object with GREATER mass will have less acceleration.