Given:
Total forest area = 52000 acres
Area of old growth trees in the forest = 0.4%
To find:
The area of old growth trees.
Solution:
We have,
Total forest area = 52000 acres
Area of old growth trees in the forest = 0.4%
Area of old growth trees is
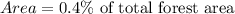
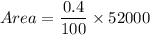
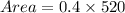
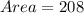
Therefore, the area of old growth trees is 208 acres.