Answer: The correct answer is option(D)
Step-by-step explanation:
All chlorine , fluorine,and bromine are members of group 17 also known as halogens.
The general electronic configuration of group 17 is written as:
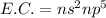 ,
,
Here 'n' is the valence shell or outer most shell present in the atom of an element.
They have fully filled s-orbital and incomplete p-orbital by one electron. There are seven valence electrons present in their valence shell. Hence the correct answer from the given options is option (D).