Answer:
Oxygen.
Step-by-step explanation:
Hello!
In this case, since the ideal gas equation allows us to compute the moles of oxygen in 5.60 L at STP (1 atm and 273.15 K) as shown below:
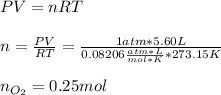
Next, given the molar mass of carbon dioxide (44.01 g/mol) we compute the moles in 10.0g of this gas via:
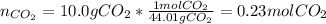
Thus, since oxygen has the greatest number of moles, we immediately infer it also has the greatest number of molecules based on the Avogadro's number.
Best regards!