Answer: The correct answer is Option 3.
Step-by-step explanation:
Saturated hydrocarbons are defined as the hydrocarbons which have single covalent C-C bonds. They are known as alkanes. The general formula for these hydrocarbons is

Unsaturated hydrocarbons are defined as the hydrocarbons which have double or triple covalent C-C bonds. They are known as alkenes and alkynes respectively. The general formula for these hydrocarbons is
 and
and

For the given options:
Option 1:

This is a saturated hydrocarbon where n = 2.
Option 2:
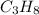
This is a saturated hydrocarbon where n = 3.
Option 3:
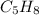
This is an unsaturated hydrocarbon where n = 5. It is an alkyne.
Option 4:
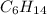
This is a saturated hydrocarbon where n = 6.
Hence, the correct answer is Option 3.