The volume of Oxygen : 20,000 L
Further explanation
Given
400 L : initial volume
150 atm : initial pressure
3 atm : final pressure
Required
Final volume
Solution
Boyle's Law
At a fixed temperature, the gas volume is inversely proportional to the pressure applied
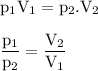
Input the value :
V₂ = (P₁V₁)/P₂
V₂ = 150 atm x 400 L / 3 atm
V₂ = 20,000 L