Answer:
28.372 grams is the mass of nugget.
Step-by-step explanation:
Density is defined as mass of the substance present in unit volume of the substance.

Mass of gold nugget = m
Volume of gold nugget = V =
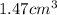
Density of the gold =
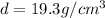
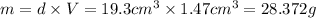
28.372 grams is the mass of nugget.