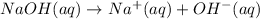Answer:
The correct answer is option A.
Step-by-step explanation:
According to the Arrhenius concept:
Acid are defined as those substance which gives hydrogen ions in their aqueous solution when dissolved in water.
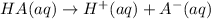
Base are defined as those substance which gives hydroxide ions in their aqueous solution when dissolved in water.

So, when NaOH were dissolved in water, it would act as a base as it will give hydroxide ion in its aqueous solution.