The question is incomplete, here is the complete question:

Determine how many grams of KI are required to produce 1.2g of

A) 0.43 g B) 0.86 g C) 1.67 g D) 3.33 g
Answer: The mass of KI needed is 0.86 grams.
Step-by-step explanation:
To calculate the number of moles, we use the equation:
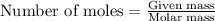 .....(1)
.....(1)
Given mass of lead (II) iodide = 1.2 g
Molar mass of lead (II) iodide = 461 g/mol
Putting values in equation 1, we get:

The chemical equation for the reaction of potassium iodide

By Stoichiometry of the reaction:
1 mole of lead (II) iodide is produced from 2 moles of KI
So,
 moles of lead (II) iodide will be produced from
moles of lead (II) iodide will be produced from
 moles of KI
moles of KI
Now, calculating the mass of KI by using equation 1, we get:
Molar mass of KI = 166 g/mol
Moles of KI =
 moles
moles
Putting values in equation 1, we get:

Hence, the mass of KI needed is 0.86 grams.