Answer:
1. 25 moles water.
2. 41.2 grams of sodium hydroxide.
3. 0.25 grams of sugar.
4. 340.6 grams of ammonia.
5. 4.5x10²³ molecules of sulfur dioxide.
Step-by-step explanation:
Hello!
In this case, since the mole-mass-particles relationships are studied by considering the Avogadro's number for the formula units and the molar mass for the mass of one mole of substance, we proceed as shown below:
1. Here, we use the Avogadro's number to obtain the moles in the given molecules of water:
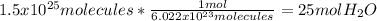
2. Here, since the molar mass of NaOH is 40.00 g/mol, we obtain:
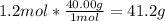
3. Here, since the molar mass of C6H12O6 is 180.15 g/mol:
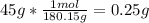
4. Here, since the molar mass of ammonia is 17.03 g/mol:
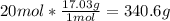
5. Here, since the molar mass of SO2 is 64.06 g/mol:
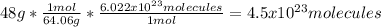
Best regards!