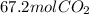Answer:- As per the question is asked, 35.0 moles of acetylene gives 70 moles of carbon dioxide but if we solve the problem using the limiting reactant which is oxygen then 67.2 moles of carbon dioxide will form.
Solution:- The balanced equation for the combustion of acetylene is:
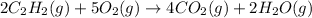
From the balanced equation, two moles of acetylene gives four moles of carbon dioxide. Using dimensional analysis we could show the calculations for the formation of carbon dioxide by the combustion of 35.0 moles of acetylene.
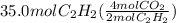
=
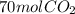
The next part is, how we choose 35.0 moles of acetylene and not 84.0 moles of oxygen.
From balanced equation, there is 2:5 mol ratio between acetylene and oxygen. Let's calculate the moles of oxygen required to react completely with 35.0 moles of acetylene.
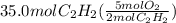
=
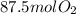
Calculations shows that 87.5 moles of oxygen are required to react completely with 35.0 moles of acetylene. Since only 84.0 moles of oxygen are available, the limiting reactant is oxygen, so 35.0 moles of acetylene will not react completely as it is excess reactant.
So, the theoretical yield should be calculated using 84.0 moles of oxygen as:
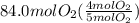
=