Answer:
For 1: The correct answer is Option D.
For 2: The correct answer is Option B.
For 3: The correct answer is Option A.
Step-by-step explanation:
For 1:
An ion is formed when a neutral atom looses or gains electrons.
- When an atom looses electrons, it results in the formation of positive ion known as cation.
- When an atom gains electrons, it results in the formation of negative ion known as anion.
We are given:
An ion carrying a charge of +3. This means that an element is loosing 3 electrons, so it has 3 valence electrons.
The elements that belong to Group 13 of the periodic table has 3 valence electrons and form +3 ions.
Hence, the correct answer is Option D.
For the given options:
Option A: Covalent bond is defined as the bond which is formed when sharing of electrons takes place between the atoms. This is usually formed between two non-metals.
Option B: Ionic bond is defined as the bond which is formed by complete transfer of electrons from one atom to another atom. The atom which looses the electron is known as electropositive atom and the atom which gains the electron is known as electronegative atom. This bond is usually formed between a metal and a non-metal.
Option C: A polar covalent bond is formed when difference in electronegativity between the atoms is present. When atoms of different elements combine, it results in the formation of polar covalent bond. For Example:
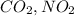 etc..
etc..
Option D: Hydrogen bonding is defined as the intermolecular force having partial ionic-covalent character. This type of bonding takes place between a hydrogen atom (attached with an electronegative atom e.g. O, N and F) and an electronegative atom (O,N and F).
Group 2 elements are considered as metals having general electronic configuration as

They will loose 2 electrons to form
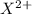 ions and thus tends to form ionic bonds.
ions and thus tends to form ionic bonds.
Hence, the correct answer is Option B.
When a positive ion gains electrons equal to the charge on the ion, the positive charge will get neutralized by the negative charge.
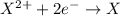
The ion will loose its charge and will form a neutral atom.
Hence, the correct answer is Option A.