Answer:
Metal : silver
Step-by-step explanation:
It is given that,
Mass of the metal, m = 0.5 kg
Temperature,
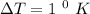
Heat, Q = 115 J
Heat absorbed is given by :


Where,
c is the specific heat of the metal


The metal having specific heat equals 230 J/kg °K is silver
Hence, this is the required solution.