Answer:
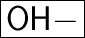
Step-by-step explanation:
According to Arrhenius concept of acid and base:
"When a base in a solution, produces/yields OH- (Hydroxide) ions."
So, when a base is dissolved in a solution, it produces OH- ions.
For example:
NaOH ⇄ Na⁺ + OH⁻ (So, it is a base)
![\rule[225]{225}{2}](https://img.qammunity.org/2023/formulas/english/college/eq413d752mwtrwwenrzwldxt4w1olmf1b3.png)
Hope this helped!
~AH1807