Answer:
Element ratio in both the compounds is same.
Hence the results are consistent with the law of definite proportion.
Step-by-step explanation:
The law of definite proportion states that the elements will combine in a definite mole ration to form compounds.
Let us calculate the moles of sodium and chlorine produced from the decomposition of the two given samples.
Sample 1:
the mass of sodium = 6.98 g
moles of sodium =
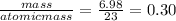
The mass of chlorine =10.7
moles of chlorine =
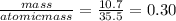
The mole ratio = 1:1
Sample 2:
the mass of sodium = 11.2 g
moles of sodium =
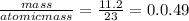
The mass of chlorine =10.7
moles of chlorine =
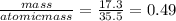
The mole ratio = 1:1
Thus in both the compounds the mole ratio is same.
Hence the results are consistent with the law of definite proportion.