Answer:
 oxygen molecules are in 22.4 liters of oxygen gas at 273 K and 101.3 kPa.
oxygen molecules are in 22.4 liters of oxygen gas at 273 K and 101.3 kPa.
Step-by-step explanation:
Pressure of the oxygen gas = P = 101.3 kPa = 1.000 atm
1 atm = 101.3 kPa
Volume of oxygen gas = V = 22.4 L
Temperature of the oxygen gas = T = 273 K
Moles of oxygen gas = n
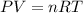 (ideal gas )
(ideal gas )

 molecules/atoms
molecules/atoms
Number of oxygen molecules : N

 molecules
molecules
 oxygen molecules are in 22.4 liters of oxygen gas at 273 K and 101.3 kPa.
oxygen molecules are in 22.4 liters of oxygen gas at 273 K and 101.3 kPa.