We, know molecular mass of water is, M = 16 g/mol .
Given mass of water, m = 18 g.
We know, number of moles is given by :
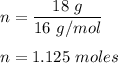
Now, 1 mole of any compound contains 6.022 × 10²³ molecules.
So, number of moles in 1.125 moles of water is 1.125 × 6.022 × 10²³ =
6.77 × 10²³ molecules.
Hence, this is the required solution.