The solution formed a precipitate when HBr is added. This indicates that the cation present would be silver ion(Ag
 ) as silver forms an insoluble precipitate with bromide ion.
) as silver forms an insoluble precipitate with bromide ion.
The equation representing the formation of silver bromide precipitate is:

Bromides of all the other cations,
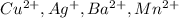 are soluble in aqueous solutions.
are soluble in aqueous solutions.
After removing the precipitate of AgBr by filtration, the supernatant solution is treated with
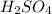 and another precipitate forms. This would be due to the presence of
and another precipitate forms. This would be due to the presence of
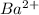 ions as barium sulfate is an insoluble precipitate.
ions as barium sulfate is an insoluble precipitate.
The equation representing the formation of barium sulfate precipitate is:
