Answer:
the effluent concentration is 0.06236 mg/L
Step-by-step explanation:
Given that;
treatment capacity
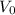 = 3,800 m³/d = ( 3,800 × 86.4) = 43.98 L/sec
= 3,800 m³/d = ( 3,800 × 86.4) = 43.98 L/sec
reactor's volume V = 45 m³ = (45 × 1000) = 45,000 L
reaction rate constant K = 0.0125 s⁻¹
influent manganese concentration
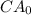 = 0.86 mg/L
= 0.86 mg/L
-
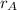 =
=
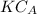
Now, performance equation for CSTR is expressed as follows;
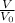 =
=
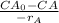
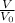 =
=
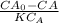
So we substitute
45000L / 43.98 L/sec = ( 0.86 mg/L - CA) / 0.0125 CA
we cross multiply
562.5CA = 37.8228 - 43.98CA
562.5CA + 43.98CA = 37.8228
606.48CA = 37.8228
CA = 37.8228 / 606.48
CA = 0.06236 mg/L
Therefore, the effluent concentration is 0.06236 mg/L