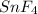Answer: In compound A, the mass ratio of Tin to Fluorine is 1 : 2
In compound B, the mass ratio of Tin to Fluorine is 1 : 4
Step-by-step explanation: For the reaction of Tin and Fluorine, the reaction follows:

To calculate the mass ratio, we simply find the mole ratio and for that we need to find the moles of Tin and fluorine respectively for each compound and then divide it by the lowest number of moles.
Molar mass Tin = 118.71g/mol
Molar mass Fluorine = 19g/mol
Formula to calculate the moles is:

For Compound A:
- Given mass of Tin = 38.5 g
Moles of Tin =

- Given mass of Fluorine = 12.3 g
Moles of Fluorine =
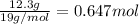
Mole ratio of Tin is =
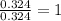
Mole ratio of Fluorine =
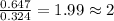
Mass ratio of Tin and fluorine is 1 : 2. The compound formed is
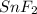
For Compound B:
- Given mass of Tin = 56.5 g
Moles of Tin =
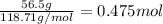
- Given mass of Fluorine = 36.2 g
Moles of Fluorine =
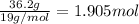
Mole ratio of Tin is =
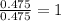
Mole ratio of Fluorine =

Mass ratio of Tin and fluorine is 1 : 24 The compound formed is