Answer:
5444.89 grams of Fe
Step-by-step explanation:
1) We need the chemical equation for Fe3O4 to find the mole ratio for Fe to Fe3O4. Chemical equation:
3Fe+4H2O → Fe3O4+4H2
The mole ratio of Fe to Fe3O4 is 3:1
2) Now, we find how many grams of Fe are needed to produce 32.5 moles of Fe3O4.
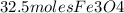
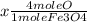 = 97.5 moles Fe needed to produce 32.5 moles of Fe3O4
= 97.5 moles Fe needed to produce 32.5 moles of Fe3O4
3) Convert 97.5 moles of Fe into grams. The molar mass of Fe is 55.845g.
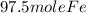
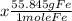 = 5444.8875g or 5444.89g.
= 5444.8875g or 5444.89g.