Answer : The mass percentage of carbon in sucrose is, 42.1 %
Explanation: Given,
Molar mass of C = 12 g/mole
Molar mass of H = 1 g/mole
Molar mass of O = 16 g/mole
First we have to calculate the molar mass of sucrose.
Molar mass of sucrose
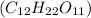 =
=
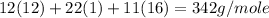
Now we have to calculate the mass of carbon in 5 g of sucrose.
As we now that there are 12 number of carbon atoms, 22 number of hydrogen atoms and 11 number of oxygen atoms.
The mass of carbon =
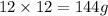
As, 342 g of sucrose contains 144 g of carbon
So, 5 g of sucrose contains
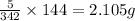 of carbon
of carbon
Now we have to calculate the mass percentage of carbon in sucrose.
Formula used :
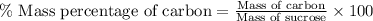
Now put all the given values in this formula, we get:
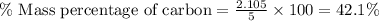
Therefore, the mass percentage of carbon in sucrose is, 42.1 %