Answer:
6.7%
Step-by-step explanation:
The formula of the compound is given as:
C₆ H₁₂ O₆
The problem here is to find the percent composition of hydrogen.
First;
Find the molar mass of C₆ H₁₂ O₆ = 6(12) + 12(1) + 6(16) = 180g/mol
Secondly;
Percentage composition of H =
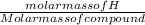 x 100
x 100
Percentage composition of H =
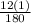 x 100 = 6.7%
x 100 = 6.7%