Answer:
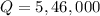 joules
joules
Step-by-step explanation:
As we know
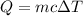
Q is the Heat Energy
M is the mass of the substance releasing /absorbing heat
C is the specific heat capacity
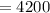 Joules per kilogram per degree
Joules per kilogram per degree
 is the change in temperature
is the change in temperature
Substituting the given values, we get
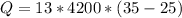
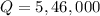 joules
joules