Step-by-step explanation:
The given data is as follows.
P = 203 k Pa, V = 2.5 L
T = 373 K, n = ?
R = 0.0821 atm L/ K mol
Convert pressure from k Pa to atm as follows.
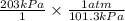 = 2.00 atm
= 2.00 atm
Now, using the ideal gas equation we will calculate the number of moles as follows.
PV = nRT
n =
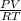
=
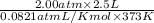
=
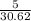 mol
mol
= 0.163 mol
Thus, we can conclude that number of moles in the given gas sample is 0.163 mol.