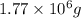Answer: The mass of copper is
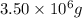 and mass of sulfur is
and mass of sulfur is
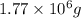
Step-by-step explanation:
We are given:
Mass of metal (Copper) = 88.39 g
Mass of non-metal (Sulfur) = 44.61 g
Mass of compound = 88.39 + 44.61 = 133 g
Mass of given sample = 5264 kg = 5264000 g (Conversion factor: 1 kg = 1000 g)
To calculate the mass of copper present in 5264 kg of sample, we use unitary method:
In 133 g of sample, the mass of copper is 88.39 g
So, in 5264000 g of sample, the mass of copper will be =
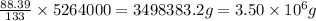
To calculate the mass of sulfur present in 5264 kg of sample, we use unitary method:
In 133 g of sample, the mass of sulfur is 44.61 g
So, in 5264000 g of sample, the mass of sulfur will be =
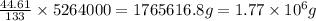
Hence, the mass of copper is
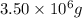 and mass of sulfur is
and mass of sulfur is