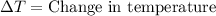Answer: There are more molecules in a gram of water.
Explanation: Specific heat is the heat required by 1 gram of a substance to raise its temperature by
 .
.
As the specific heat of water (4.186 J/g°C) is more than that of ethanol (2.450 J/g°C) it means more energy is required to raise the temperature of a gram of water by 1°C. Which means the molecules are tightly bound and thus more molecules are contained in a gram of water.

Q= heat gained
m= mass of the substance
c = heat capacity