Answer:
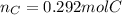
Step-by-step explanation:
Hello,
In this case, by means of the given mass, we should remember that one mole of a particular element, equal its atomic mass. In such a way, since carbon is the involved element and its molar mass is 12g/mol, we perform the following mathematical operation in order to obtain the moles via proportions:
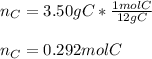
Best regards.