Answer:
The density of the nugget is 19.3g/cm³
It is gold because all samples of gold will have the same density value
You should buy it.
Step-by-step explanation:
Given parameters:
Density of gold = 19.3g/cm³
Length on all sides = 5cm
Mass in kg = 2.413kg
Unknown:
Density = ?
Is it gold = ?
Should you buy it = ?
Solution:
To solve this problem, we need to find the density of the nugget first.
Density =
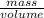
Mass = 2.413kg
We need to convert to g:
1kg = 1000g
2.413kg = 2413g
Volume = L³ = 5³ = 125cm³
Therefore;
Density =
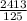 = 19.3g/cm³
= 19.3g/cm³
The density of the nugget is 19.3g/cm³
It is gold because all samples of gold will have the same density value
You should buy it.