Answer:- pH = 1.17
Solution:- HBr is a strong acid and KOH is a strong base. So, it's a strong acid vs strong base titration.
For this type of titration, if we have equal moles of acid and base then solution is neutral and the pH will be 7.
If the moles of base are more than moles of acid then solution will be basic and pH will be more than 7.
And if the moles of acid are more than moles of base then solution will be acidic and the pH will be less than 7.
So, let's calculate the moles of acid and base and see which one is in excess.
HBr and KOH reacts in 1:1 mol ratio which is clear from the below balanced equation:
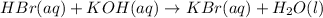
To calculate the moles we convert mL into L and multiply with the molarity.
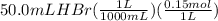
= 0.0075 mol HBr
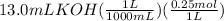
= 0.00325 mol KOH
moles of HBr are more than moles of KOH. So, excess moles of HBr
= 0.0075 - 0.00325
= 0.00425 mol
Total volume of the solution will be the sum of the volumes of acid and base added.
So, total volume = 50.0 mL = 13.0 mL = 63.0 mL = 0.0630 L
Molarity of excess HBr =
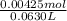
= 0.0675 M
HBr is the strong acid so hydrogen ion concentration will be same as the concentration of excess HBr that is 0.0675 M.
So, the pH of the solution = - log(0.0675)
pH = 1.17