Answer:
The amount of water converted from liquid to gas with 6,768 joules is approximately 3.035 g
Step-by-step explanation:
The amount of heat required to convert a given amount of liquid to gas at its boiling point is known as the latent heat of evaporation of the liquid
The latent heat of evaporation of water, Δ
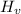 ≈ 2,230 J/g
≈ 2,230 J/g
The relationship between the heat supplied, 'Q', and the amount of water in grams, 'm', evaporated is given as follows
Q = m × Δ
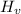
Therefore, the amount of water, 'm', converted from liquid to gas at the boiling point temperature (100°C), when Q = 6,768 Joules, is given as follows;
6,768 J = m × 2,230 J/g
∴ m = 6,768 J /(2,230 J/g) ≈ 3.035 g
The amount of water converted from liquid to gas with 6,768 joules = m ≈ 3.035 g.