Answer: The mass of copper(II)sulfate are, 89.6 grams
Explanation: Given,
Molecules of
 =
=

Molar mass of
 = 160 g/mole
= 160 g/mole
First we have to calculate the moles of
 .
.
As,
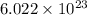 number of
number of
 atoms present in 1 mole of
atoms present in 1 mole of

So,
 number of
number of
 atoms present in
atoms present in
 mole of
mole of

Now we have to calculate the mass of



Therefore, the mass of copper(II)sulfate are, 89.6 grams