Answer: Family 16 is most likely to accept 2 electrons.
Step-by-step explanation:
Family 16 has the electronic configuration
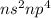 . As to attain stability the p orbital will readily accept 2 electrons.
. As to attain stability the p orbital will readily accept 2 electrons.
Therefore, family 16 is most likely to accept 2 electrons. Family 16 is also known as chalcogens.
Whereas, family 2 has the electronic configuration
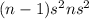 . As the s orbital is completely filled so there is no need to accept 2 electrons.
. As the s orbital is completely filled so there is no need to accept 2 electrons.
Family 12, and family 18 has the electronic configuration
![[Ar] nd^(10) ns^(2)](https://img.qammunity.org/2017/formulas/chemistry/high-school/8ixujs3re8ww5fparlsjazzsy1kzaii36j.png) and
and
![[He]ns^(2) np^(6)](https://img.qammunity.org/2017/formulas/chemistry/high-school/grh2ad4jsjh5hyssfnlx69469wh3tl0r3e.png) respectively.
respectively.
Both family 12 and 18 has completely filled orbitals so it will not accept any electrons.