Answer: The correct answer is Option B.
Step-by-step explanation:
STP conditions are defined as standard temperature and pressure conditions.
- The temperature at this condition is taken as 273.15 K
- The pressure at this condition is taken as 1 atm or 101.3 kPa or 760 mmHg or 760 Torr.
Also,
1 mole of a gas occupies a volume of 22.4 L
The value of gas constant at this condition is taken as
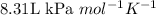 ,
,
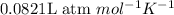 etc..
etc..
Hence, the correct answer is Option B.